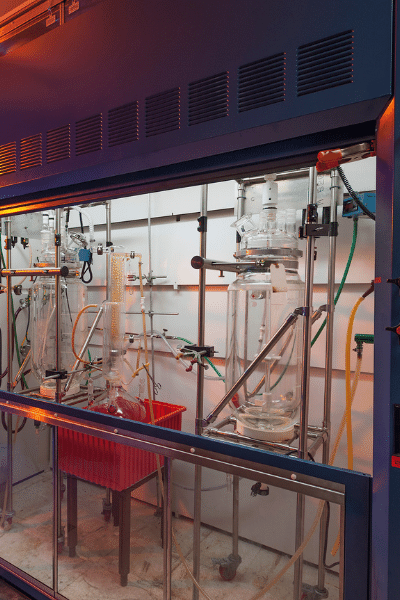
Operation Facilities

Manufacturing
We provide the manufacture & supply of pharmaceutical intermediates and Active Pharmaceutical ingredients (APIs) on an integrated manufacturing model. We specialized in contract manufacturing & exclusive manmufacturing of pharmaceutical intermediates and advanced intermediates. We are one of the most reliable manufacturer of Active Pharmaceutical Ingredients (APIs) based on loan licensing. Our state of art manufacturing facilities are fully compliant to cGMP & approved by State FDA. We everyday on mission to build strong products which give consistant quality, therefore our scientist, operational team & engineers are working with most advanced machineries & equipments to support our one quality for all concept. Our facilities are capable to deliver products from kilogram scale to ton scale. Our all facilities independently handle more than 300 KL reactions capacity per month.
- Glass Line Reactors 1 KL to 6.5 KL
- Stainless steel reactor 1 KL to 6 KL
- Fluid bed dryer (FBD)
- Tray Dryer
- Utilities supported with Chiller, Colling tower, Brequete & Gas fire boiler etc..
- Centrifuges (CF)
- ETP
- Hydrogenator Etc..
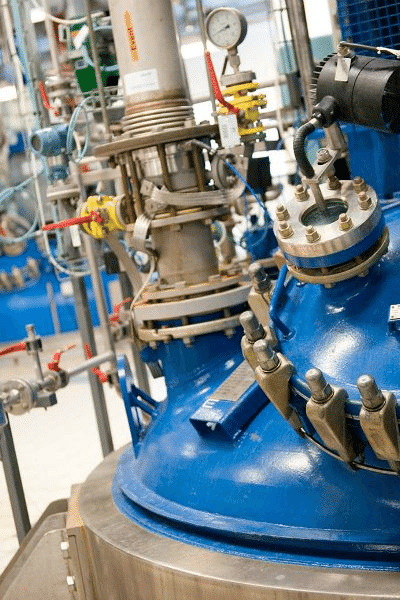

Quality
We are all responsible for quality at our facility.
Throughout the development of all our products, our Quality Management Team makes sure that they are of the highest quality, purity, efficacy and safety.
Quality Assurance team ensures that the latest updates in Good Manufacturing Practices (GMP) are being translated into Standard Operating Procedures (SOPs). The quality units at the respective sites further ensure that these SOPs are implemented to consistently deliver excellent products.
In addition to this, the manufacturing sites are regularly audited by Quality Assurance to verify 24×7 compliance.We work towards continuous improvement of our Quality Management System (QMS) and all its elements. Organizational structure, procedures, processes, and resources should be part of the quality management system. In addition, it should include activities to build confidence that the API will meet its intended specifications for quality and purity.
We are building and maintaining a strong culture of quality through the on-going development, training, and empowerment of our personnel.

Research & Development
We have been providing pharmaceutical intermediate and API process development services for nearly three decades, from our network of R&D and manufacturing facilities across India.
We have a team of 10 process chemists and engineers, supported by an infrastructure of fume-hoods, kilo labs, state-of-the-art analytical instrumentation, along with non-GMP production areas.
Process Development Services
- Process/route selection
- Manufacturing support
- Solvent CC reduction
- Yield improvement
Analytical Services
- Analytical method development and transfe
- Analytical method validation
- Reference standard qualification
- Testing of raw materials
- Intermediates and finished product testing
- Third-party sample analyses
- Wet chemistry and compendial tests
Additional Services
- Impurities and reference Standards
- Documentation